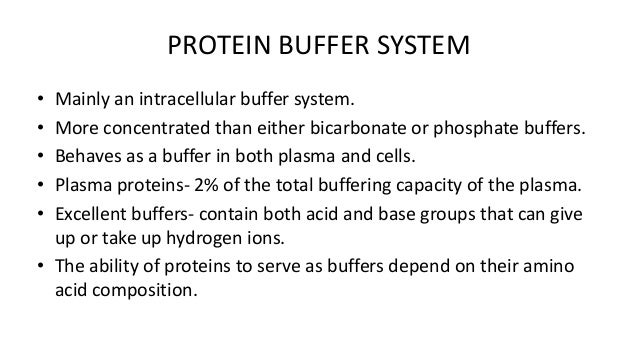
Plasma Protein Buffer System
Plasma protein buffer system. A very quick overview of how proteins and individual amino acids can modify the pH of a solution. Ad Beautiful Multicolor Images. There are three buffer systems.
Samples were stored at 80 C until. It can bind to small amounts of acid in the blood helping to remove that acid before it changes the bloods pH. Stain Live or Fixed Cells.
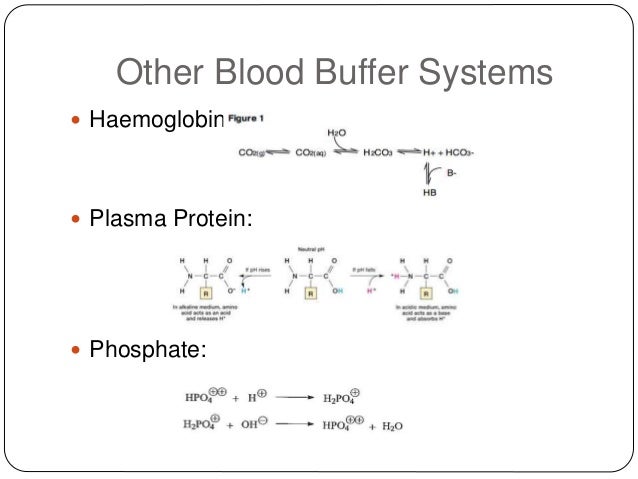
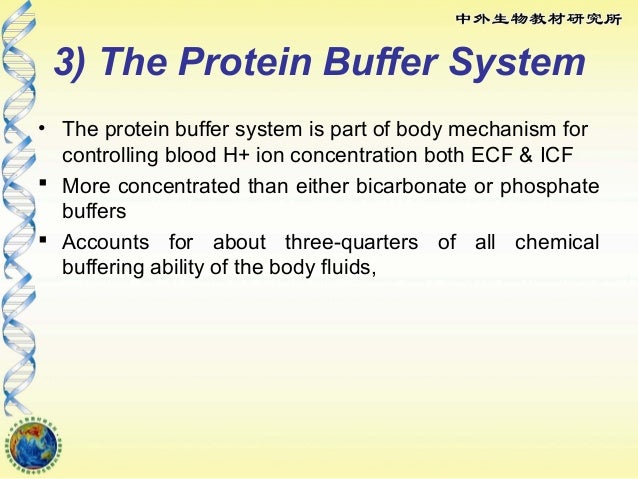
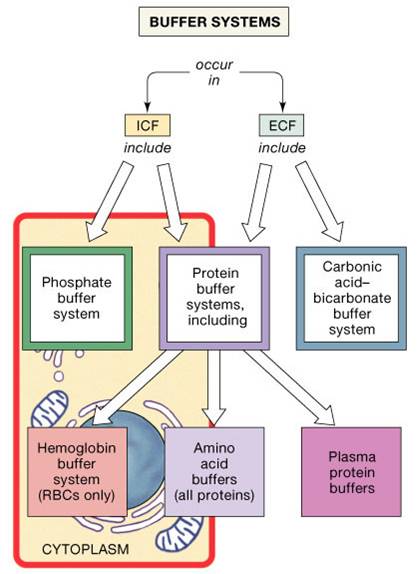
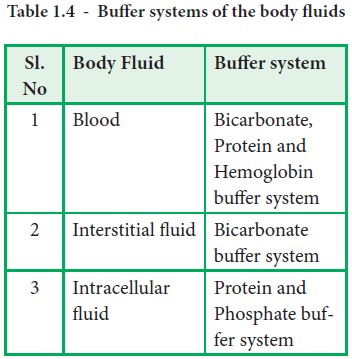
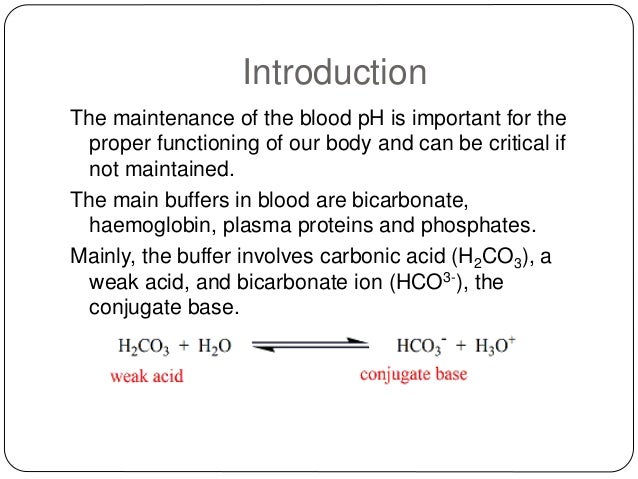
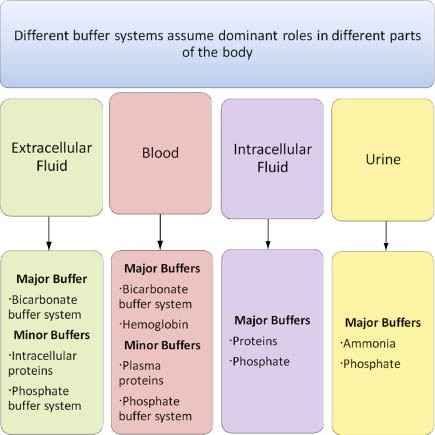
The blood buffers consists of the plasma proteins hemoglobin oxy-hemoglobin bicarbonates and inorganic phosphates. When pH of ECF decreases cells pumps out Hydrogen out of the ECF and into the ICF where the intracellular proteins can buffer them. Stain Live or Fixed Cells.
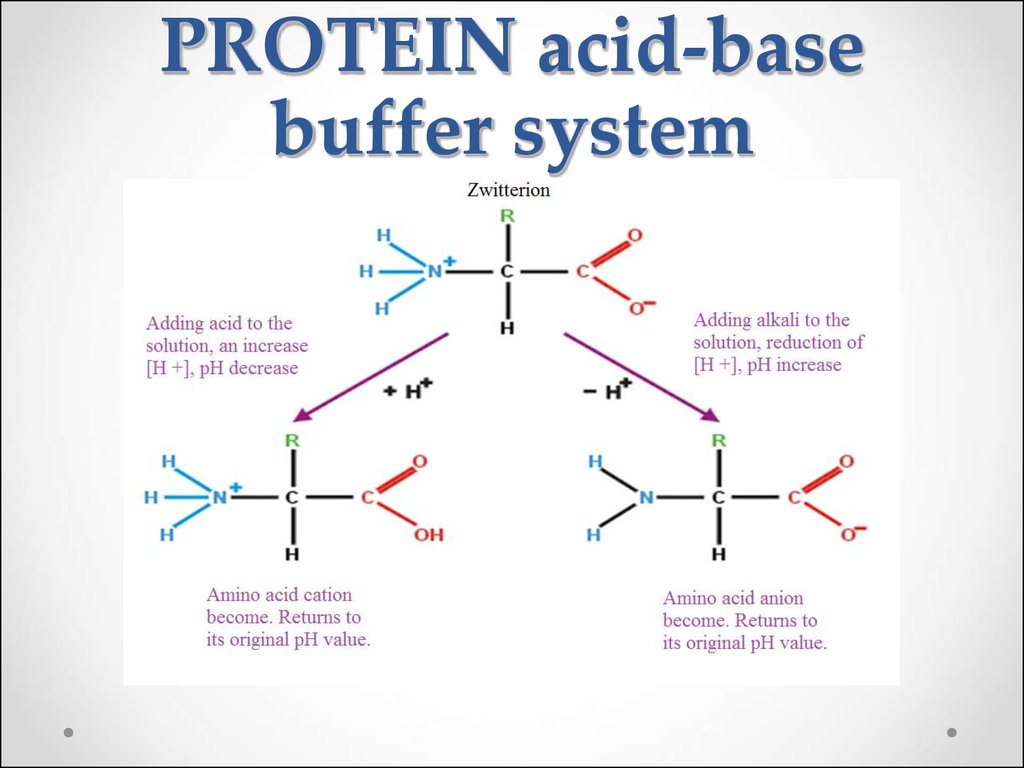
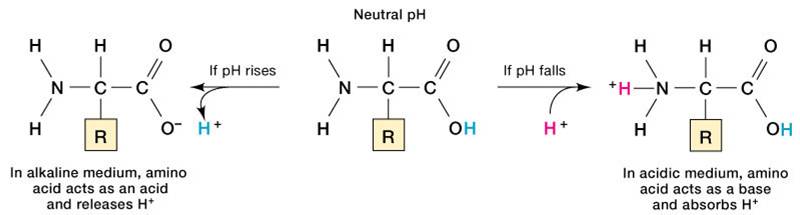
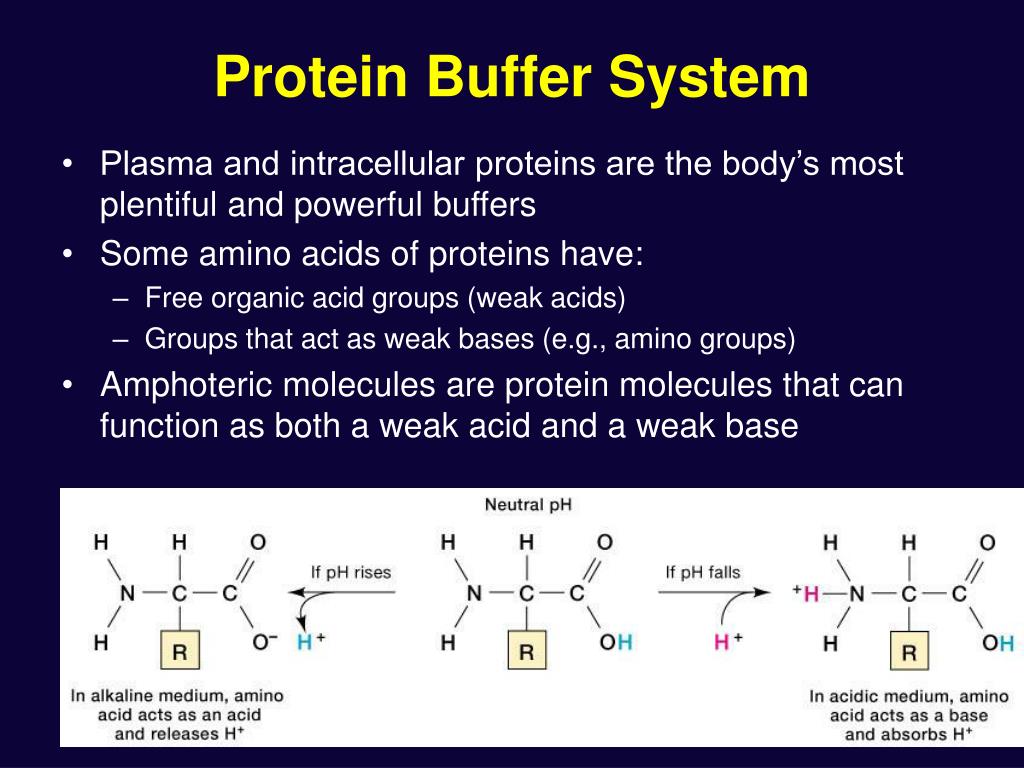
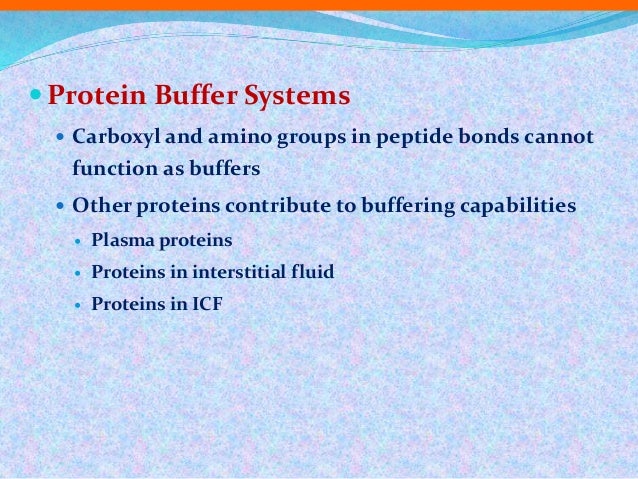
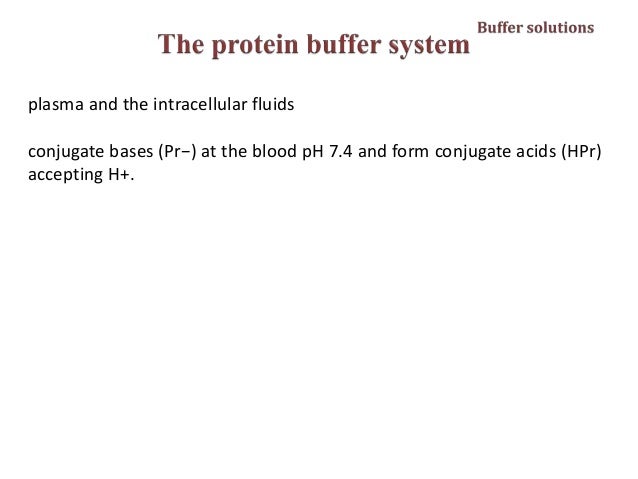
Contd The carboxyl and amino groups are what enable proteins to act as buffers. Pro-tein concentrations were analyzed using a bicin-choninic acid BCA protein assay kit Pierce. Protein buffer system phosphate buffer system and bicarbonate buffer.
Pr β pH pI where Pr is the concentration of plasma proteins in grams per liter β is the buffer value of plasma proteins in millimoles per gram per pH unit pH is the ECF pH and pI is the isoelectric point of plasma proteins. Many other proteins act as buffers as well. Three main buffers are bicarbonate haemoglobin and phosphate.
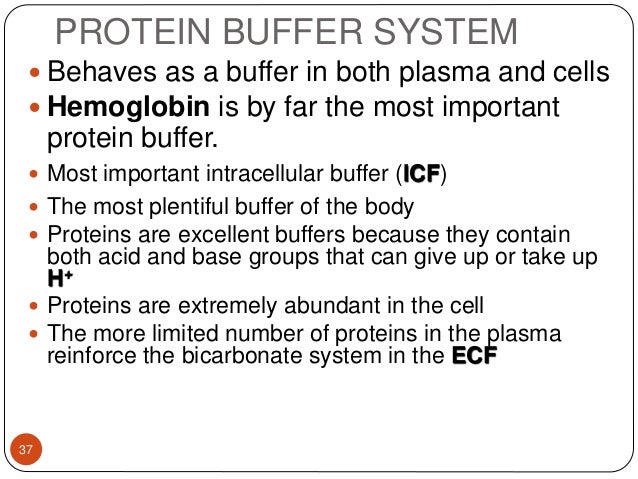
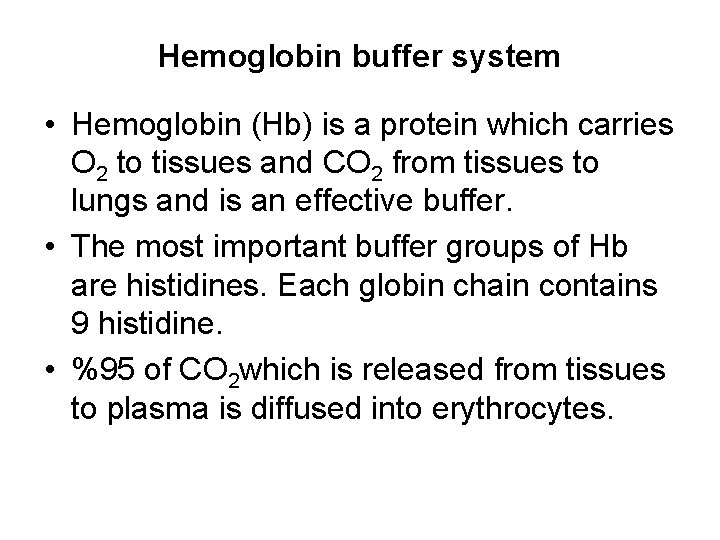
ATP AMP ADP zInorganic phosphate. Plasma proteins are responsible for protein buffer system. Hemoglobin as a Buffer.
The protein hemoglobin makes an excellent buffer. The buffer systems functioning in blood plasma include plasma proteins phosphate and bicarbonate and carbonic acid buffers.
ATP AMP ADP zInorganic phosphate.
The most important buffer groups of proteins are imidazole groups of histidine pK about 73 and each albumin contains 16 histidines. Ad Beautiful Multicolor Images. PROTEIN BUFFER SYSTEM 37 Behaves as a buffer in both plasma and cells Hemoglobin is by far the most important protein buffer. Protein especially albumin accounts for greater proportion 95 of non bicarbonate buffer in plasma. Nearly all proteins can function as buffers. When the pH of the ECF increases pumps in plasma membranes move H out of the ICF and into the ECF. Contd The carboxyl and amino groups are what enable proteins to act as buffers. Pr β pH pI where Pr is the concentration of plasma proteins in grams per liter β is the buffer value of plasma proteins in millimoles per gram per pH unit pH is the ECF pH and pI is the isoelectric point of plasma proteins. ATP AMP ADP zInorganic phosphate.
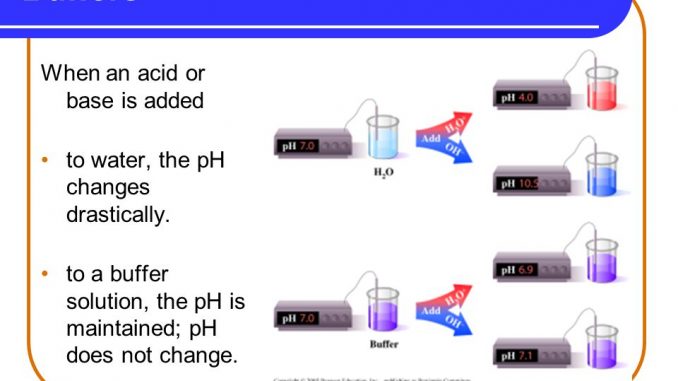
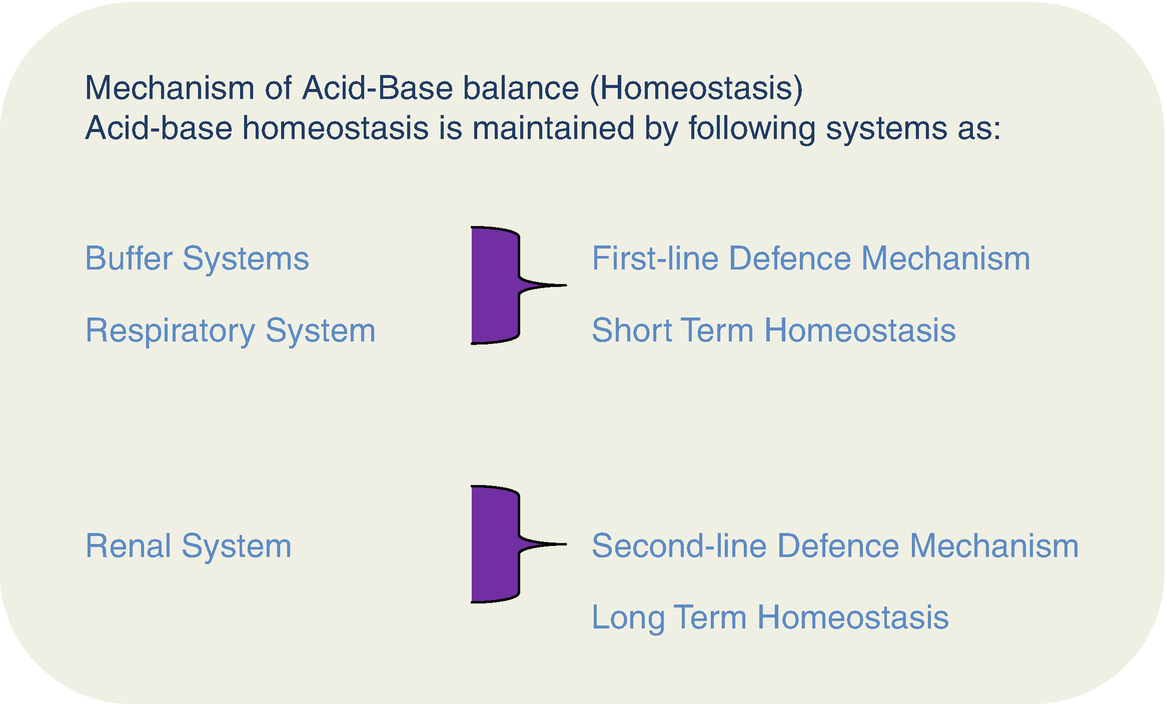
What is the primary buffer in the plasma. Pr β pH pI where Pr is the concentration of plasma proteins in grams per liter β is the buffer value of plasma proteins in millimoles per gram per pH unit pH is the ECF pH and pI is the isoelectric point of plasma proteins. Hemoglobin as a Buffer. A very quick overview of how proteins and individual amino acids can modify the pH of a solution. When pH of ECF decreases cells pumps out Hydrogen out of the ECF and into the ICF where the intracellular proteins can buffer them. The pH of the extracellular fluid including the blood plasma is normally tightly regulated between 732 and 742 by the chemical buffers the respiratory system and the renal system. Buffer systems help to maintain constant plasma pH.

























Post a Comment for "Plasma Protein Buffer System"